Urgent Product Defect Correction in Australia (Recall for Product Correction in New Zealand)
Philips Sleep and Respiratory Care Devices – Australia and New Zealand
Understand the steps we take to ensure the safety of your replacement CPAP or BiPAP device
February 21, 2023
While we work to provide replacement devices as quickly as possible, we want to share the steps we are taking to ensure your First Generation DreamStation replacement device is safe to use so you can be confident in your new device.

Replacement devices, whether it is a newly manufactured or reworked device, contain a silicone foam. Please note that the update to the foam is applicable to all Philips replacement CPAP, BiPAP and ventilator devices.
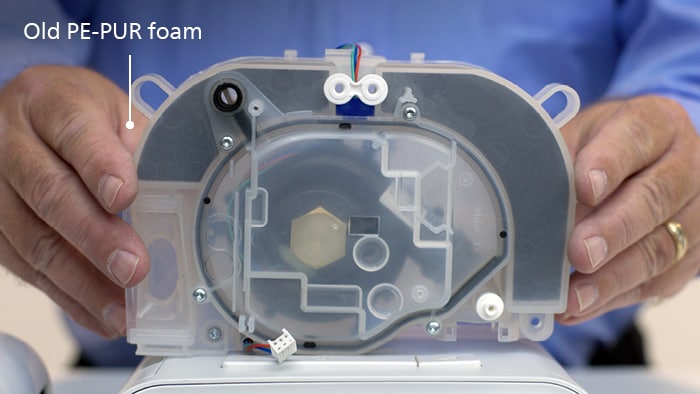
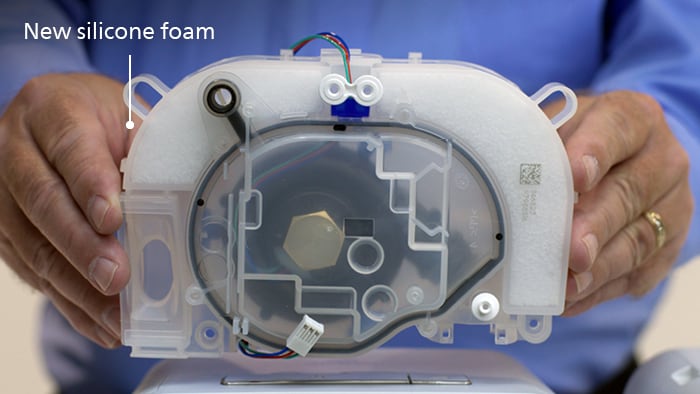
Affected devices contained a gray polyester-based polyurethane, or PE-PUR, sound abatement foam component, which helped to reduce the noise coming from your device. Replacement devices will include a white silicone foam.
Replacement devices will come with a 2-year Philips product warranty or, Philips will honour the unexpired Philips product warranty period of your affected device; whichever is longer.
Our goods and services come with guarantees that cannot be excluded under the Australian and New Zealand Consumer Law.
Your rights under the Australian and New Zealand Consumer Law are in addition to any remedy the local Philips entity may provide you.
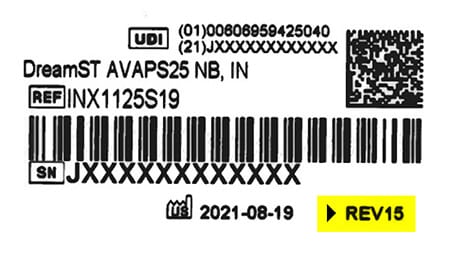
All remediated devices will include a label at the bottom showing REV15 or higher. This indicates that the device has been remediated to include silicone foam.
Please note that the color of the label may vary depending on the type of replacement device.

Regular device cleaning and maintenance is important for your therapy
Over time, the filter of your replacement device may change color due to environmental factors like pollution, dust, and smoke. This change in color is normal.


We advise that you change your device filters as instructed in your device manual.
Please do not replace the internal foam, or any parts of your affected device, yourself. Trying to open or repair your device yourself or using a third-party repair kit could interfere with your device and may stop it from working completely.
Philips Respironics does not offer repair kits for sale. Only authorized service professionals can perform the rework.

Need further assistance?
Please contact the Philips on 1800 830 517 in Australia or 0800 796 515 in New Zealand for more assistance.
